 400-9988-900
400-9988-900
 400-9988-900
400-9988-900
The global manufacturing industry is entering the era of Industry 4.0, yet automation in pharmaceutical equipment remains relatively underdeveloped.
Automation is no longer a “nice-to-have” feature—it has become the foundation of future competitiveness for pharmaceutical manufacturers.
As a leading representative of China’s pharmaceutical equipment industry, Truking Technology is empowering pharmaceutical companies to transform their factories through intelligent equipment and integrated automation systems.

According to the latest data, the global pharmaceutical market is projected to reach USD 1.9 trillion by 2027, with a compound annual growth rate (CAGR) of 6–8%.
Despite continuous market expansion, the industry still faces multiple structural bottlenecks. Traditional models can no longer meet the new demands of competition and development, and the need for transformation has never been more pressing.
Uneven automation maturity:
Many manufacturers are still operating at the level of standalone equipment automation, where each production stage functions independently with little coordination. For instance, in solid dosage production, unpacking, weighing, feeding, and intermediate product transfer rely heavily on manual labor, driving up workforce costs and operational pressure.
Data silos and traceability challenges:
Paperbased batch records are still common, often leading to human errors and omissions, which in turn make quality traceability difficult. Batch record review may take several days to weeks, reducing production efficiency and exposing companies to compliance risks.
Outdated supply chain and warehouse management:
Conventional warehouse management is inefficient, with inaccurate inventory control and poor batch tracking, failing to meet modern GSP requirements for full traceability and precision management throughout the drug lifecycle.
Global competition and cost pressure:
Intensified international competition and the costreduction measures of healthcare systems (such as volumebased procurement) require pharmaceutical companies to achieve unprecedented levels of efficiency and cost optimization, accelerating the need for automation and digital transformation.

Advancing automation and digitalization is not merely a technical upgrade—it is a strategic imperative for survival and growth in a fiercely competitive market.
Enhanced quality consistency and reduced human error:
By replacing manual operations with automated robots and intelligent inspection systems, manufacturers can minimize human contamination risks and significantly improve production accuracy and batch consistency.
Optimized operational efficiency and lower overall cost:
Automated systems enable continuous production with realtime quality monitoring, automatically removing defective intermediates. Industry cases show that smart transformation can improve production efficiency by 30% and reduce labor costs by 40%.
Strengthened compliance and data integrity:
Through systems such as MES, SCADA, and WMS, automation and digitalization enable full data traceability—from raw materials to finished products—helping companies meet FDA and EMA inspection standards and reducing audit preparation time from several weeks to just a few days.
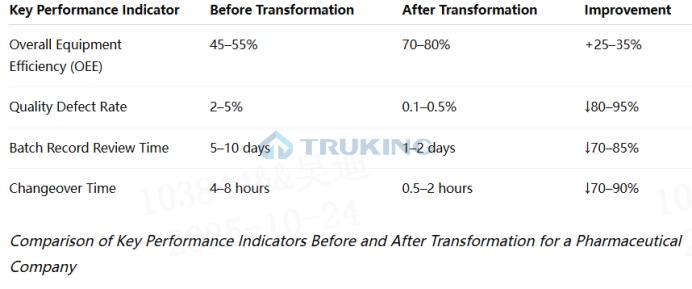

Implementing Industry 4.0 in the pharmaceutical sector is a systematic transformation process, requiring coordinated progress across architecture, technology, and strategic dimensions.
The core architecture of an intelligent manufacturing system consists of four layers—equipment, control, business management, and enterprise management—achieving endtoend integration from data acquisition at the device level to strategic decisionmaking at the top.
Equipment Layer:
Comprising process equipment, utility systems, and laboratory instruments, this layer integrates PLCs, HMIs, sensors, actuators, and online PAT testing at the raw material stage, enabling realtime data collection and equipment control.
Control Layer:
Industrial automation platforms such as SCADA and DCS form the backbone of process monitoring and control, delivering precise parameter management, automated recipe control, and comprehensive equipment supervision.
Business Management Layer:
Centered on MES and QMS, and supported by LIMS, WMS, DMS, and TMS, this layer connects warehouse, production, and operational systems to ensure endtoend process synchronization and realtime visibility.
Enterprise Management Layer:
Platforms such as ERP, CRM, and SRM, combined with advanced BI analytics, support strategic decisionmaking, resource optimization, and integrated business planning, providing toplevel visibility across the organization.
Implementation should follow the principle of “overall planning with phased execution and priority to urgent needs.”
First, deploy core automation equipment and systems (ERP, SCADA) to establish a solid data foundation and digitalize production processes.
Next, build the operational information framework centered on MES and QMS, supported by WMS and LIMS.
Finally, integrate data platforms, BI tools, and AI applications to enable predictive maintenance and holistic optimization—ultimately constructing an IT infrastructure that supports enterprisewide digital operations.

In the pharmaceutical industry’s journey toward Industry 4.0, the smart automated warehouse has evolved from an optional feature to a central hub.
It is not just a storage space, but a strategic node that synchronizes material, information, and value flows—addressing the industry’s challenges in efficiency, cost, compliance, and traceability.
Revolutionary space utilization:
Highrise storage racks extend storage vertically, increasing space efficiency by 3–5 times compared to conventional flat warehouses and significantly reducing land costs.
Unmanned operation for cost and efficiency gains:
Automated stacker cranes, shuttles, robots, conveyors, and AGVs revolutionize inbound/outbound and picking processes, cutting labor dependency while improving throughput by over 50% and virtually eliminating human error.
Uncompromised compliance and traceability:
WMS and WCS systems fully comply with FDA, EU GMP, GMP, and GxP regulations, precisely managing material information, batch numbers, expiry dates, and status. Every item carries a complete electronic data record, enabling full bidirectional traceability from finished product to raw material.
Driving supply chain collaboration:
By deeply integrating with ERP, realtime inventory data drives procurement, production, and sales planning, greatly enhancing supply chain responsiveness and resilience.
Case Example:
To cope with market growth and improve supply chain responsiveness, one pharmaceutical company built an intelligent logistics center that covers the entire process from raw materials to production, warehousing, and shipment.
Its core objectives were unmanned operation, smart decisionmaking, and highefficiency logistics turnover. The WMS system, informed by TMS data, preplans vehicle dock assignments. Upon arrival, goods are unloaded onto telescopic conveyors, shaped, and automatically or manually labeled. Robotic palletizers then stack the goods, which are transferred via conveyor lines or AGVs to inbound points.
The system automatically assigns storage locations for unmanned or lowlabor warehousing. According to production schedules, AGVs deliver materials and packaging to designated lines on a JIT basis. Overhead transport systems deliver materials directly and precisely to their workstations, ensuring uninterrupted production flow.
Under WCS control, stacker cranes coordinate all automated devices for fast retrieval and transfer within highbay racks. The WMS system optimizes storage layout and task allocation, integrating seamlessly with ERP, MES, TMS, and serialization systems to eliminate data silos. After packaging, robotic palletizers stack finished goods, which are transported via AGV or conveyor systems to the warehouse. Telescopic conveyors load goods directly into trucks, enabling highefficiency outbound logistics.

The completed facility achieved automatic transfer and storage of 52,000 raw and auxiliary material units, intelligent finished product handling, and endtoend logistics automation, marking a benchmark in pharmaceutical Industry 4.0 transformation.
Automation marks the turning point from “manufacturing” to “smart manufacturing” in the pharmaceutical industry. Industry 4.0 is not merely a technological evolution—it is a complete rethinking of management philosophy.
Truking Technology will continue to leverage its expertise in intelligent manufacturing and system integration to help pharmaceutical enterprises build safer, more efficient, and greener factories of the future.
