 400-9988-900
400-9988-900
 400-9988-900
400-9988-900
In pharmaceutical manufacturing, discussions often revolve around highprofile equipment such as filling lines, lyophilizers, and isolators. Yet the pharmaceutical water system—though rarely in the spotlight—is the invisible cornerstone of every aseptic process.
Whether it’s Water for Injection (WFI), Purified Water (PW), or Pure Steam (PS), even minor deviations can lead to batch failures, validation breakdowns, or costly production shutdowns.
Within Truking Technology’s ecosystem, Truking Watertown has long specialized in the R&D, manufacturing, and validation of pharmaceutical water and purification systems. Its Kingene Architecture is not merely a piece of equipment—it’s a complete engineering philosophy that redefines the reliability, validation logic, and maintainability of pharmaceutical water systems.
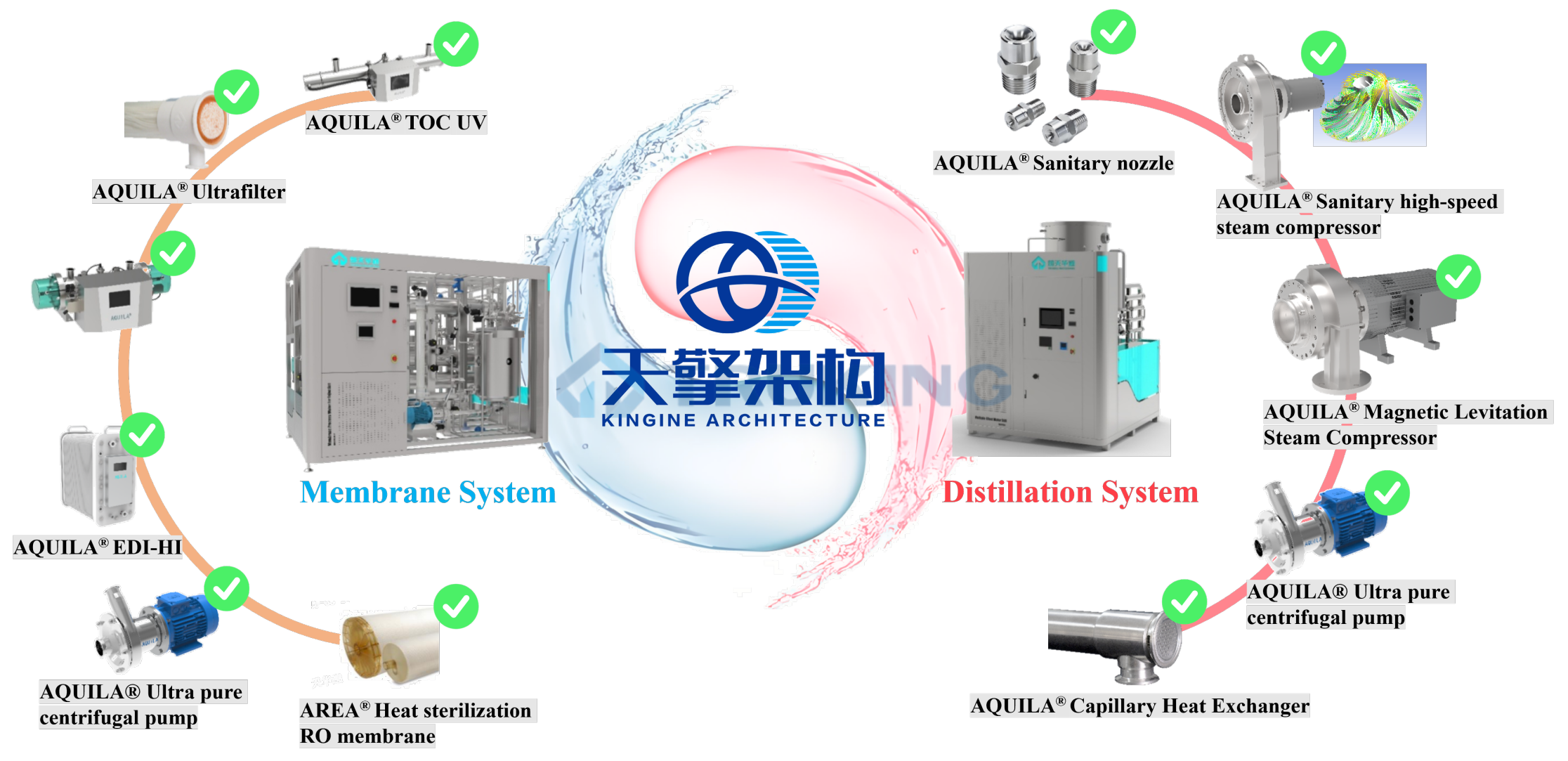
Traditional water systems are often assembled like patchwork: one reverseosmosis unit here, one EDI there, a storage tank, and then a loosely connected control system.
While this approach may suffice for small projects, it struggles under the complexity of modern GMP facilities—multiple interfaces, lengthy validation cycles, high energy consumption, and difficult maintenance. The Kingene Architecture introduces a modular and integrated design concept that transforms the water system from a collection of components into a unified engineering solution. All functional modules—pretreatment, RO, doublestage RO, EDI, pure steam generation, and storagedistribution—are standardized with unified interfaces and clear logic.
For pharmaceutical manufacturers, this brings three major benefits:
-Shorter project timelines: modules are prevalidated at the factory (FAT), requiring only onsite assembly and commissioning.
-Higher system stability: thermal sanitization, deadleg control, and flow balance are considered from the design stage.
-Stronger scalability: additional modules can be integrated quickly when production expands.
In essence, Kingene Architecture elevates water systems from “equipment integration” to a fully validated system engineering platform.
The Unseen Craft Behind Stability
In pharmaceutical water systems, true quality lies not in appearance but in engineering precision. Every detail of Kingene Architecture reflects this rigor:
-RO membranes and EDI modules: built with internationalstandard components, achieving water resistivity above 18 MΩ·cm.
-Tanks and piping: made of SUS 316L stainless steel with electropolished surfaces (Ra ≤ 0.4 μm).
-Thermal sanitization: automatic, chemicalfree Pasteurization cycles ensure microbiological control per GMP standards.
-Welding and inspection: orbital welding with full traceability and 100% weld inspection.
-Valves and instruments: globally certified brands for seamless automation integration.
These microlevel controls form the invisible foundation for the system’s longterm reliability.

Validation and Compliance in the Data Integrity Era
As global regulators such as the FDA and EMA tighten their focus on data integrity, Truking Watertown’s system is designed not just to “run,” but to be verified, traced, and analyzed.
The control platform combines a scalable PLC + SCADA architecture supporting:
-Continuous parameter monitoring (temperature, conductivity, TOC, flow, pressure, etc.);
-Automated alarms and event logs compliant with ALCOA+ principles;
-User access hierarchy and electronic signatures per 21 CFR Part 11;
-Remote monitoring, reporting, and auditready data review.
This transforms the water system into a digital quality backbone that regulators can trust.
From “HighPurity Water” to “Green Water”
Water systems have traditionally been seen as energyintensive and wasteheavy. Kingene Architecture integrates sustainability at the design level to achieve highpurity production with lower environmental impact.
Key features include:
-RO concentrate recovery, raising total water recovery above 70%;
-Lowpressure membranes and variablefrequency pumps, cutting energy use by 15–25%;
-Distributed heat recovery, recycling thermal energy for tank insulation and sanitization;
-Smart flushing cycles, extending membrane life and reducing chemical cleaning.
The result is a system that meets not only water quality standards but also energy efficiency and ESG compliance benchmarks.
Reliability Is Verifiability
In pharmaceutical engineering, the ultimate value of equipment lies in being verifiable.Kingene Architecture follows the VModel validation framework—from URS (User Requirements) through DQ/IQ/OQ/PQ, ensuring full traceability.Every component specification, welding record, and test result is documented within a structured validation chain.This documentdriven approach makes audits and revalidations faster, transparent, and stressfree.

True Quality Is the Stability You Don’t See
Within every pharmaceutical plant, the water system functions like the body’s circulatory system—quiet yet vital, sustaining product integrity and manufacturing continuity.
Truking Watertown’s Kingene Architecture embodies a mature engineering mindset that redefines the technological boundary of pharmaceutical water systems.
It is not a showcase of complexity, but a validated, trustworthy, and sustainable system—a tangible step in China’s transformation from manufacturing to engineering and digital excellence in pharmaceutical technology.
