 400-9988-900
400-9988-900
 400-9988-900
400-9988-900
On July 7, 2025, the European Commission and PIC/S released the latest revision of GMP, marking the first inclusion of artificial intelligence within the regulatory framework. The update sets clear boundaries: dynamic models, such as generative AI and large language models, cannot be directly applied in critical GMP contexts, while only static, deterministic models are permitted. Just as importantly, AI systems must remain verifiable, controllable, and ensure data integrity.
Visual inspection machines sit at the core of pharmaceutical quality control, and their performance directly determines both defect detection rates and regulatory compliance. Many industry players still rely on unsupervised learning methods, which compare products to a limited set of “normal” samples. However, these approaches often prove insufficient, as they often miss subtle or complex defects, produce high false positives due to batch variations, and lack the transparency required for GMP compliance.
Truking Technology takes a different approach by combining supervised learning with AIGC-based data enhancement.
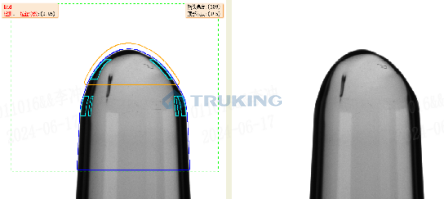
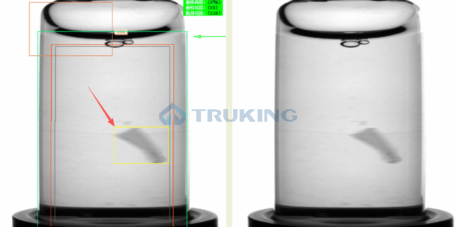
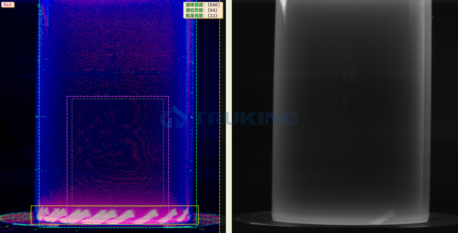

With this approach, Truking’s inspection machines achieve superior performance in detection accuracy, stability, and regulatory verifiability compared to unsupervised alternatives.
For customers, the benefits are clear:
As AI becomes an integral part of GMP regulation, manufacturers face the challenge of balancing innovation with compliance. Truking Technology is already leading this transformation—delivering solutions that not only meet regulatory requirements but also advance pharmaceutical quality inspection into a smarter, more reliable future.
